08 Jun
Misconceptions About Running Clinical Trials Outside the U.S.

Deciding where and who will manage your clinical trial will have a direct impact on its success. This is an important, complex decision, and many factors must be considered.
Often bigger, more well-known global CROs are chosen, but only for these reasons alone. Factors like flexibility, location, approval timelines, and participant populations are not closely examined enough.
The misconceptions surrounding using smaller CROs outside of the U.S. could be why many studies are not finding much success.
Bigger is better?
Big businesses use their size to create the illusion that they are the safest option. However, this sometimes means trials get crushed under their weight.
Like any sizeable company, big business means the sacrifice of customer service, the management of studies, and flexibility.
Dealing with big global CROs also means dealing with more significant problems. These companies are constantly growing with global acquisitions, which can result in frequent disruptions and complex handovers. These internal disruptions can then cause major delays and complications for trials.
While it may seem like these global giant CROs are the best option, it is the smaller to midsized CROs that are truly thriving, in part because of their size.
Smaller CROs are not always undergoing constant changes like the bigger ones, allowing them to remain more focused on the trials at hand. Their smaller scale means more availability for hands-on and attentive customer service.
These boutique companies are also not held down by concrete processes like bigger CROs and can be more agile and innovative in their problem-solving. Instead of having cookie-cutter solutions, small CROs can more easily adapt and respond to unique issues.
There is also the perception that using a global CRO with all in-house services means systems are more streamlined and less risky. Smaller CROS will outsource high-risk, specialised services to a network of trusted experts. While the communication itself will be no different as you’re are dealing with completely different departments anyway, boutique CROs provide noticeably better service. Contractors are only as good as their last job, whereas underperformance is often lost in larger CROs.
When choosing a CRO, you should also consider the size of the trial itself. Large-scale trials may be more suitable for the big global CROs, while the smaller trials run the risk of getting lost in the shuffle. In addition to this, those big CROs may not prioritise smaller trials.
Australia and New Zealand, however, are more apt to managing these smaller sized trials.
Cost and time effective
Clinical trials alone are a considerable expense, so wherever costs can be saved, they .
Due to generous tax incentives, running a clinical trial in Australia can be up to 60% cheaper compared to the U.S. The Australian Government offers a return of up to 43.5% for every dollar spent on R&D for global trial sponsors[1].
These incentives aside, New Zealand and Australia are significantly less in comparison to the US and other parts of the world.
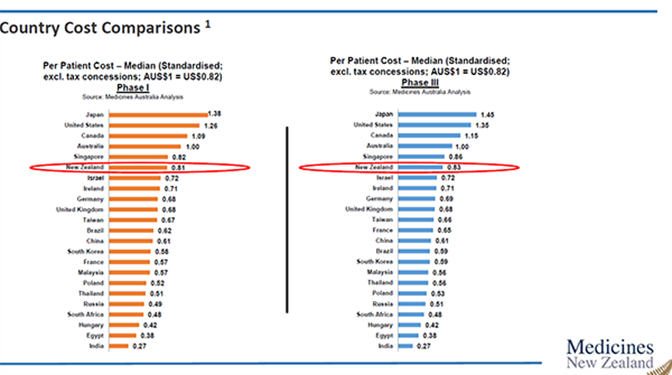
(Source: Medicines Australia (2014). Clinical trial metrics: benchmarking Australia's performance)
Delays play a major role in trial costs, often resulting in millions of wasted dollars. These delays are frequently due to lengthy regulatory approvals and poor patient recruitment and retention.
Australia and New Zealand have one of the fastest regulatory approval environments in the world. Only one ethics submission is required in New Zealand, and trials can achieve rapid start-up in 35 days. Due to this speed, consider Australia and New Zealand for early start up and get other global destinations up later. This allows streamlining of any issues before global rollout.
New Zealand and Australia also do not require a Pre-Investigational New Drug submission like in the U.S. This immediately cuts out the significant amount of time spent dealing with the U.S. Food and Drug Administration trying to facilitate approval so that a trial can move forward. If a trial is conducted in Australia and New Zealand, it can be initiated in parallel to the preparation of the US .
Participant recruitment and retention
There is an idea that New Zealand is small, which it is in comparison to the U.S. However, as we have seen with large CROs, bigger does not always mean better.
As many experienced sponsors will know, participant recruitment and retention are an ongoing issue with clinical trials. This issue has not only been known to cause delays, but it has also completely derailed some studies.
These regions have strong economies, a skilled multilingual workforce, and a diverse population, making them ideal locations for clinical studies.
PharmaSols has had first hand experience of this kind of recruitment speed. In one case, we were able to recruit twice as many patients as our global CRO partner.
Pandemic lockdowns
The pandemic’s effects are still rippling across the globe, interfering with nearly every aspect of our daily lives.
U.S. government restrictions have made patient recruitment even more of a challenge. Moreover, lockdowns have imposed considerable delays and interferences to trials. However, New Zealand and Australia’s ongoing management of the virus is something worth noting.
In New Zealand, clinical trials have been classed as essential, which means that regardless of COVID alert levels, studies will continue as usual.
Telehealth capabilities and virtual trial technologies have been widely embraced in this region, with early and quick adoption. Our remote monitoring SOPs take into consideration the safety of the sites, patients, and staff, while also ensuring the continuity of trials.
Get in touch with us to see how we can help ensure the success of your trial.
Sources:
[1] Report: Australia 60% cheaper than US for clinical trials, https://www.outsourcing-pharma.com/Article/2016/10/26/Report-Australia-60-cheaper-than-US-for-clinical-trials, Accessed 05/05/2021
[2] ANZCTR , Completed Commercial Trials, AU NZ 01/01/2015 - 31/12/2020, target v actual patients
Other News
March 2024 (1)
February 2024 (1)
December 2023 (1)
November 2023 (1)
October 2023 (1)
September 2023 (2)
August 2023 (1)
July 2023 (1)
June 2023 (2)
May 2023 (3)
April 2023 (1)
March 2023 (2)
The Go-to region for clinical trials (1)
HiRO – our global advantage, tailored solutions and key partnerships (1) (1)
HiRO – an emerging full-service global CRO (1)
HiRO – Top CRO in APAC 2022 (1) (1)
November 2022 (1)
October 2022 (1)
September 2022 (1)
August 2022 (1)
July 2022 (1)
June 2022 (1)
May 2022 (1)
April 2022 (1)
March 2022 (1)
January 2022 (1)
December 2021 (1)
November 2021 (1)
October 2021 (2)
September 2021 (2)
August 2021 (3)
July 2021 (3)
June 2021 (2)
May 2021 (1)
April 2021 (2)
March 2021 (1)
February 2021 (1)
December 2020 (5)
November 2020 (1)
October 2020 (5)
September 2020 (1)
August 2020 (2)
May 2020 (5)
January 2024 (0)



