
HiRO: Just One of the Heroes in Clinical Research Management
12 March 2024
You’ve probably heard it said before: people are the greatest assets. A company that fails to recognise the value of their people miss the greatest opportunities for success and future growth.

How To Prevent Clinical Drug Trial Delays
14 February 2024
Delayed clinical drug trials are a thorn in the side of any study stakeholder. This blog will discuss at the main causes of delayed trials, and what precautions can be taken to ensure timelines can be adhered to.

How Does Rapid Start Up Affect Clinical Trials?
16 January 2024
Fast, efficiently run trials benefit from gaining a competitive edge, a good reputation and credibility amongst stakeholders, as well as a period of exclusivity if the product is the first to be introduced to market.

Ethnicity And Clinical Trials - Where Is the Diversity?
14 December 2023
One of the most important aspects of a clinical trial is that it represents a diverse ethnic profile. For a pharmaceutical product to be accepted by international regulatory bodies such as the FDA and the EMA, it is essential that the patient recruitment process places ethnic diversity as a priority.

The Impact Your CRO Choice Has on The Quality of Your Trial
16 November 2023
Quality is paramount in clinical trials, as it ensures that the data collected is reliable and that the trial results are meaningful. However, achieving quality can be challenging, given the many factors that can impact the success of a trial.

How to Execute a Cost-effective and Efficient Clinical Trial
20 October 2023
Clinical trials play an indispensable role in the life sciences sector, serving as the cornerstone for determining the safety and efficacy of new medical interventions.
The stakes are high; inefficient management can lead to considerable delays and increased costs. According to industry research, 45% of trials finish behind schedule.

The Benefits of Rapid Start Up and How to Achieve It
12 September 2023
Time is often always a factor in the development of new therapies, especially for conditions with unmet medical needs or during global health emergencies like we saw with the pandemic.

PharmaSols CRO Dedicated Study Start-Up Team – Taking Trial Sites From Selection to Initiation Faster
15 September 2023
Meet Jorrit Sipkema – Director Study Start-up at PharmaSols CRO, a HiRO company. Jorrit has worked over 20 years in clinical research in AsiaPac and Europe for Pharmaceutical companies, CRO's and Central Laboratories. His experience includes clinical operations, project management and quality assurance both at management and operations levels.

Clinical Trials in ANZ: Harnessing Population Diversity for Recruitment
10 August 2023
Recruitment and retention of trial participants can often be a critical bottleneck in clinical research. It is widely accepted that almost half of all delays in clinical trials are a result of problems with patient recruitment and retention.
When it comes to the success of clinical trials, patient recruitment and retention plays a pivotal role.

Accelerating FDA Approvals – It’s Faster Outside the US
10 July 2023
Although it may seem counterintuitive, obtaining FDA approval is often expedited when the clinical trials are conducted outside the US.
This process acceleration isn't about bypassing thorough checks or compromising on quality. Instead, it leverages the benefits of certain regions' clinical research ecosystems, like those of Australia and New Zealand (ANZ).

Advanced Therapeutics in Australia
05 June 2023
Australia is a recognised internationally as a Go-To- Destination for clinical research and the conduct of global clinical trials.

Accelerating Clinical Trials: Australia and New Zealand's Streamlined Processes Attract Biotechs Worldwide
14 June 2023
By leveraging the fast approval timelines and utilizing local contract research organizations (CROs), sponsors can achieve rapid study start-up and obtain high-quality data accepted by major regulatory bodies, ultimately saving time and costs associated with clinical trials.

Innovative Strategies For Successful Drug Development
11 May 2023
We are living in an extraordinary time of scientific innovation, where our understanding of biology is exploding and our ability to improve human health is unprecedented with new opportunities to develop new innovative medicines and devices.

ANZ Region Rescues Stalled Global Clinical Trials
11 May 2023
Randomized, controlled trials are considered the gold standard for evaluating the safety and efficacy of potential new medicines and therapies, as they provide a rigorous and unbiased way to compare treatments to placebo or standard care.

Global Pharma can accelerate commercial sales and registration in China through Real World Data
08 May 2023
Hainan Boao Lecheng special zone is the first area in China where RWD can be widely used. The Lecheng Real World Study (RWS) pathway allows overseas drugs, medical devices, and IVDs with clinical urgency status, that have not been approved by the China NMPA, to be sold and used in real world clinical settings in the Hainan province, China.

ANZ – Cost-Effective solutions for clinical trials
05 April 2023
Australia and New Zealand have risen to be the go-to region to conduct clinical trials for global sponsors due to rapid study start-up timelines, high-quality data generation, proven participant recruitment and retention, and cost-effectiveness.

PharmaSols’ Global Team of Clinical Trial Experts
14 March 2023
Clinical trials are complex and expensive, and the ability to keep a trial on track saves money, time, and resources for the sponsor. Each trial is unique, and things don’t always go to plan. CROs must have the expertise to manage any situation that arises.

Save R&D Time and Cost - Webinars
03 April 2023
Watch the sessions from the recent Harvest Integrated Research Organisation (HiRO) event in Taipei.
Hear what our partners said about how the Australian region can be leveraged to deliver rapid, cost-effective, quality clinical trial solutions.
Specialists from our partners speak: 360biolabs, a BioAgilytix company, Nucleus Network and Acclime Australia.

The Go-to region for clinical trials
08 February 2023
The ANZ region has become the go-to region for biotechs to conduct their clinical trials. That’s not surprising as the region generates high-quality clinical data while being time and cost-effective.

HiRO – our global advantage, tailored solutions and key partnerships
20 December 2022
Last year we announced that PharmaSols was acquired by global CRO, Harvest Integrated Research Organisation (HiRO) – recently announced as the Top CRO in the APAC region. Through HiRO, we were able to offer a larger global network, stronger leadership and investment in market-leading integrated systems and processes.

HiRO – an emerging full-service global CRO
12 December 2022
Last year we announced that PharmaSols was acquired by global CRO, Harvest Integrated Research Organisation (HiRO). This meant we were able to offer a larger global network, stronger leadership and investment in market-leading integrated systems and processes. This month we will discuss a bit more about our parent company HiRO – their values, vision, service offerings, partnerships, and end-to-end specialised clinical trial solutions.

HiRO – Top CRO in APAC 2022
06 December 2022
The world is witnessing an enormous demand for innovative medicines to manage and cure diseases. Despite the explosive record demand levels and best efforts of pharma companies during the COVID-19 pandemic, there is still uncertainty regarding the fastest approval process defined by the FDA.

ANZ Trends Upwards in Popularity for Clinical Trials
08 November 2022
Australia and New Zealand are considered the go-to place to conduct clinical trials for an increasing number of global sponsors. That’s not surprising as the region generates high-quality clinical data while being time- and cost-effective.

ANZ – Destination to speed up your clinical trial
20 October 2022
Before recruiting patients for a clinical trial, the study goes through a study start-up phase which includes processes like ethics and regulatory approval & site identification/onboarding. Ethics and regulatory processes are seen as a key limiting factor in the study start-up phase of a clinical trial.

Australia and New Zealand – the haven for Patient Recruitment and Retention in Clinical Trials
15 September 2022
Patient recruitment and retention are critical for the success of any clinical trial. It is widely accepted that almost half of all delays in clinical trials are a result of problems with patient recruitment and retention.

Quality, Speed and Cost – Where can clinical trials deliver all three?
30 August 2022
Australia and New Zealand are considered as the go-to place to conduct clinical trials for an increasing number of global sponsors. That’s not surprising as the region generates high quality clinical data while being time- and cost-effective.

How biotechs are using streamlined Ethics and Regulatory processes in ANZ to accelerate clinical trials
21 July 2022
We’ve always heard about the saying, time is money. You might wonder how this applies to clinical trials.

How Biotechs can utilise Australia and New Zealand to extend seasonal clinical trials and rescue studies
14 June 2022
Randomised, controlled trials are considered the gold standard in assessing the safety and effectiveness of potential new medicines and therapies.
The Effect of the Pandemic on locations for conducting clinical trials for Biotechs
18 May 2022
Through the spring of 2020, it became evident that the Covid-19 pandemic would have a dual impact on the clinical research sector. While it has provided the opportunity to conduct research for vaccines, treatments, and diagnostic tests for COVID-19; it has also acted as a major disruptive force, halting trials in their tracks, and exacerbating existing fragilities within the sector.

Global Clinical Trials take advantage of significant Australian R&D tax incentives
22 April 2022
Australia and New Zealand are well-established regions for international clinical trials. Global sponsors understand the region’s ability to deliver quality research outcomes; rapid start-up, recruitment and participant retention, streamlined ethics and regulatory pathways, internationally accepted quality data from world-leading research facilities and Investigators.

PharmaSols’ Global Solution
08 March 2022
PharmaSols is the oldest and best-connected privately-owned Clinical Research Organisation in the Pacific region. Having successfully supported patient needs and delivered outstanding clinical trial results for more than 20 years, our addition to the global group HiRO in November 2021 means we now provide flexible end-to-end integrated solutions to sponsors around the globe.

Directly attributing our success to the quality of our team
17 January 2022
Having grown to be one of the leading CROs in Australia and New Zealand, we directly attribute our success to the quality of our team.

PharmaSols - HiRO announcement
01 December 2021
PharmaSols acquired by the newly established global CRO based in Shanghai, HiRO.

The ANZ Advantage
18 November 2021
4 reasons why biotechs need to reconsider running clinical trials in the US – the ANZ advantage
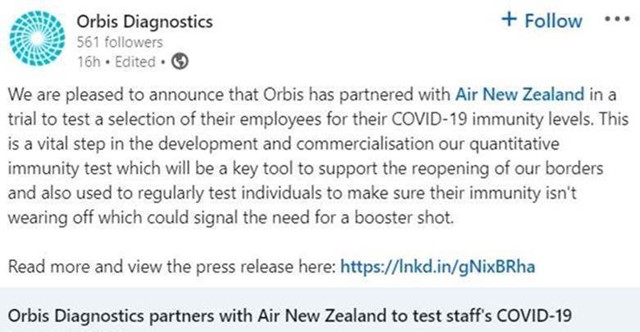
PharmaSols proud to be the NZ CRO partner on an important COVID-19 clinical trial.
28 October 2021
PharmaSols proud to be working with Orbis Diagnostics as the NZ CRO partner on this important COVID-19 clinical trial.

ANZ Region – place to extend seasonal clinical trials and rescue studies
15 October 2021
The timelines for clinical trials are often disrupted or face challenges that risk their success for various reasons. However, some types of trials rely on seasons, meaning they are racing to meet nature’s own timeline.
So, what happens to a study if it doesn’t meet the deadline?

PharmaSols Partnering with Molecular Templates, Inc.
30 September 2021
PharmaSols is delighted to be partnering with Molecular Templates, Inc. (MTEM) on their phase 1b study of MT-5111 in advanced human epidermal growth factor receptor 2 (HER2)-expressing solid tumours across multiple sites in Australia and New Zealand.

How To Avoid Costly Errors in Ethics and Regulatory Submissions
08 September 2021
Ethics and Regulatory applications are a necessary complexity that can determine trial start-up timeframes or, in some cases, throw trial startup timelines immediately off-target if done incorrectly.
Selecting who should be responsible for completing this application can also have a significant impact on the continuity of the trial.

Assured Continuity
19 August 2021
Yesterday New Zealand was dealt a new challenge with one suspected case of the new Delta variant in the community. With the New Zealand government’s commitment to eradication of COVID-19 in the community, New Zealand has been placed in a lockdown for to a 7 day period. However we have proven our ability to work through the challenges of running a clinical trial through a pandemic.

RADIO NEW ZEALAND NATIONAL_Valneva New Study
18 August 2021
Exciting for PharmaSols CRO and NZ to be involved in this international COVID-19 vaccine trial.

What to Look for in a CRO Partner
09 August 2021
There are many factors that can lead to the derailment of a clinical trial. Your Clinical Research Organisation (CRO) partner shouldn’t be one of them.
Understanding the importance of different regional benefits, individual organisations' responses to the pandemic, and their size can help this critical decision.

#NewTrialAnnouncement
22 July 2021
PharmaSols is delighted to be working with UNITY Biotechnology, Inc. (UNITY) on their latest ophthalmologic study UBX1325-02, across multiple sites in Australia and New Zealand.

The Key to Increasing Patient Retention in Clinical Trials
13 July 2021
There are a lot of reasons a trial can be derailed, with poor patient retention being among one of the biggest struggles.
It is natural for some patients to drop out, as study participation is voluntary. However, identifying the common reasons for participant drop out helps sponsors to address these issues to increase the patient retention rate.

We are Finalists for the Amcham-DHL-Express 'Success and Innovation Awards Exporter of the Year to the USA' $1million to $10million!
09 July 2021
The American Chamber of Commerce in New Zealand is pleased to announce the finalists for the 2021 AmCham-DHL Express Success and Innovation Awards, the 21st year of these awards celebrating success and innovation for companies doing business with the USA.

OUTSOURCING-PHARMA.COM _ Australia & New Zealand Appealing to Clinical Researchers: Greenphire
14 June 2021
Thanks in part to swift COVID-19 response and relatively low infection rates, more sites and sponsors are looking at the region to locate their studies.

Misconceptions About Running Clinical Trials Outside the U.S.
08 June 2021
Deciding where and who will manage your clinical trial will have a direct impact on its success. This is an important, complex decision, and many factors must be considered.

Welcome to PharmaSols
13 May 2021
Check our video - after months of hard work and planning, we are thrilled to announce our new brand and our new website!

The Cost of Delaying A Trial
12 April 2021
From discovery and development through to market, the life cycle of new medicine is a profoundly complex process, frequently met with obstacles. Research produced by PhRMA found that clinical trials take on average six to seven years to assess safety and efficiency, with the overall probability of success less than 12%.

Quality Assurance: The Key to Every Successful Clinical Trial
17 March 2021
Quality is an essential element that underpins every aspect of a clinical trial. As an industry, we easily understand the benefits of quality monitoring to ensure quality data and clinical trial management. However, we often underestimate or do not understand the broader Quality Assurance (QA) function and how a well-trained team of QA auditors can ensure successful trial outcomes.

How to Run A Trial During a Pandemic
16 February 2021
When the pandemic hit, it was like someone stuck a stick through the wheels of a bike, and we all went toppling over. Everyone had the intention to keep moving, keep peddling forward, but the uncertainty of COVID-19 felt like a heavy burden.

PHARMACEUTICAL TECHNOLOGY _ New Zealand: A Promising Destination For Trials in A Post-Covid-19 World?
18 December 2020
Unlike the rest of the world, New Zealand’s speedy and decisive response to Covid-19 has successfully enabled the country’s clinical trials to continue largely unscathed. Allie Nawrat talks to key actors in the New Zealand clinical space about how Covid-1.

Successful Patient Recruitment In a COVID-19 World
03 December 2020
Pharmaceutical Solutions (PSL), which is New Zealand and Australia-based, has been geographically fortunate, and our proven strategies have also ensured the continuation and success of our clinical studies.

STUFF.CO.NZ _ Overseas medical researchers eye up NZ for clinical trials
03 December 2020
Overseas medical researchers eye up NZ for clinical trials.
Jacquie Palmer, managing director of Pharmaceutical Solutions speaks to Stuff about New Zealand being the ideal destination for clinical trials.

Rapid Study Startup and High Patient Recruitment for US Biotech Clinical Trial
01 December 2020
A US-based biotech company was seeking a CRO who could successfully recruit a large number of elderly patients in New Zealand. A challenge squeezed into a three-month recruitment window.

NBR _ COVID brings boom in clinical trials [New Business Review]
01 December 2020
“It’s quite exciting times for the industry, and how well New Zealand has dealt with Covid has really opened up some opportunities."
New Zealand’s Covid-free status is attracting more US biotech and pharmaceutical companies than ever to run clinical trials here.
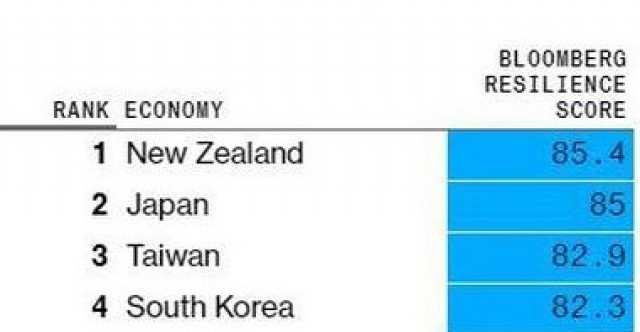
BLOOMBERG _ New Zealand's COVID-19 response rated best in the world
25 November 2020
New Zealand is the best place in the world to be in the coronavirus era, according to the new Bloomberg Covid resilience ranking.

BetterLife Pharma chooses Pharmaceutical Solutions CRO for Australia coronavirus trial of AP-003
27 October 2020
The company has selected Pharmaceutical Solutions Ltd, which specializes in managing clinical trials in Australia and New Zealand.

Our Managing Director Jacquie Palmer was recently interviewed by Outsourcing Pharma
20 October 2020
Our Managing Director Jacquie Palmer was recently interviewed by Outsourcing Pharma on New Zealand’s growing clinical trials industry and response to Covid-19.

“I wanted to be part of the solution to Covid-19”: Michele Ashby, Executive Director, US
20 October 2020
We’re incredibly proud of our Executive Director of Business Development in the United States, Michele Ashby, who is taking part in a US based COVID-19 vaccine trial. Hear from Michele, her insights as a clinical trial participant.

Pharmaceutical Solutions: Quality Assurance in Clinical Trials
15 October 2020
With international travel restrictions in place, many pharmaceutical/biotech/CRO companies have had to place quality assurance audits for their clinical trials on hold.

Assured Continuity of Clinical Trials in Australia and New Zealand during the Covid-19 pandemic
09 October 2020
Confidence in the Australian and New Zealand clinical environment has been shown globally with 170 new study start-ups registered on the ANZCTR between April and August 2019

2020 Finalist - Westpac Business Awards
07 September 2020
Pharmaceutical Solutions is honoured to be selected as a finalist in the Westpac Auckland Business Awards - Excellence in International Trade category.

Clinical trial continuity in NZ: Optimal Clinical Trials
17 August 2020
Clinical trials are considered an essential service by the New Zealand Government and so can continue to operate at all alert levels

Moving forward, together
13 August 2020
After 102 days, New Zealand has recorded its first new cases of community transmission of Covid-19. As of midday on Wednesday 12th Aug, there are only four cases confirmed, all of which are in Auckland.

STOCKTALK _ Biotechs set to boom with clinical trial floodgates open
05 June 2020
A fantastic discussion about Australia’s clinical trial industry.
Jacquie Palmer speaks with the heads of ASX-listed Dimerix Limited and Cynata Therapeutics about how Covid-19 has propelled trial methods forward, and the implications of the pandemic on the future of the sector.
NEWSTALK ZB _ Call for NZ clinical trials after Govt's $37m vaccine spend
27 May 2020
Listen to Managing Director Jacquie Palmer discuss New Zealand’s clinical research potential on NewstalkZB this morning. We’re excited to start a valuable discussion about NZ’s capabilities and opportunities in the clinical sector.

NZ HERALD.CO.NZ _ COVID-19 and New Zealand’s clinical research sector
27 May 2020
"In the global race to find a vaccine, we need populations that have had little exposure to Covid-19 and New Zealand can offer a vital link in the global effort to halt the novel coronavirus," said the group's managing director, Jacquie Palmer.”

MEDICINES NZ _ Clinical trials in NZ worth $1.2 billion
21 May 2020
New Zealand has a window of opportunity to increase market share.
Chief executive of Medicines NZ, Graeme Jarvis, says there has to be a collaborative effort between the industry, clinical research sector and government to make New Zealand a bigger player on the world stage.

Medicines NZ report estimates clinical trials worth $1.2b over 5 years
20 May 2020
A new report independently commissioned by Medicines New Zealand estimates that since 2013, clinical trials of prescription medicines have contributed at least $1.2 billion to the New Zealand economy and have benefitted the health system and Kiwi patients.